Patient-reported outcomes in chronic hepatitis delta
Patient-reported outcomes in chronic hepatitis delta: An exploratory analysis of the Phase III MYR301 trial of bulevirtide
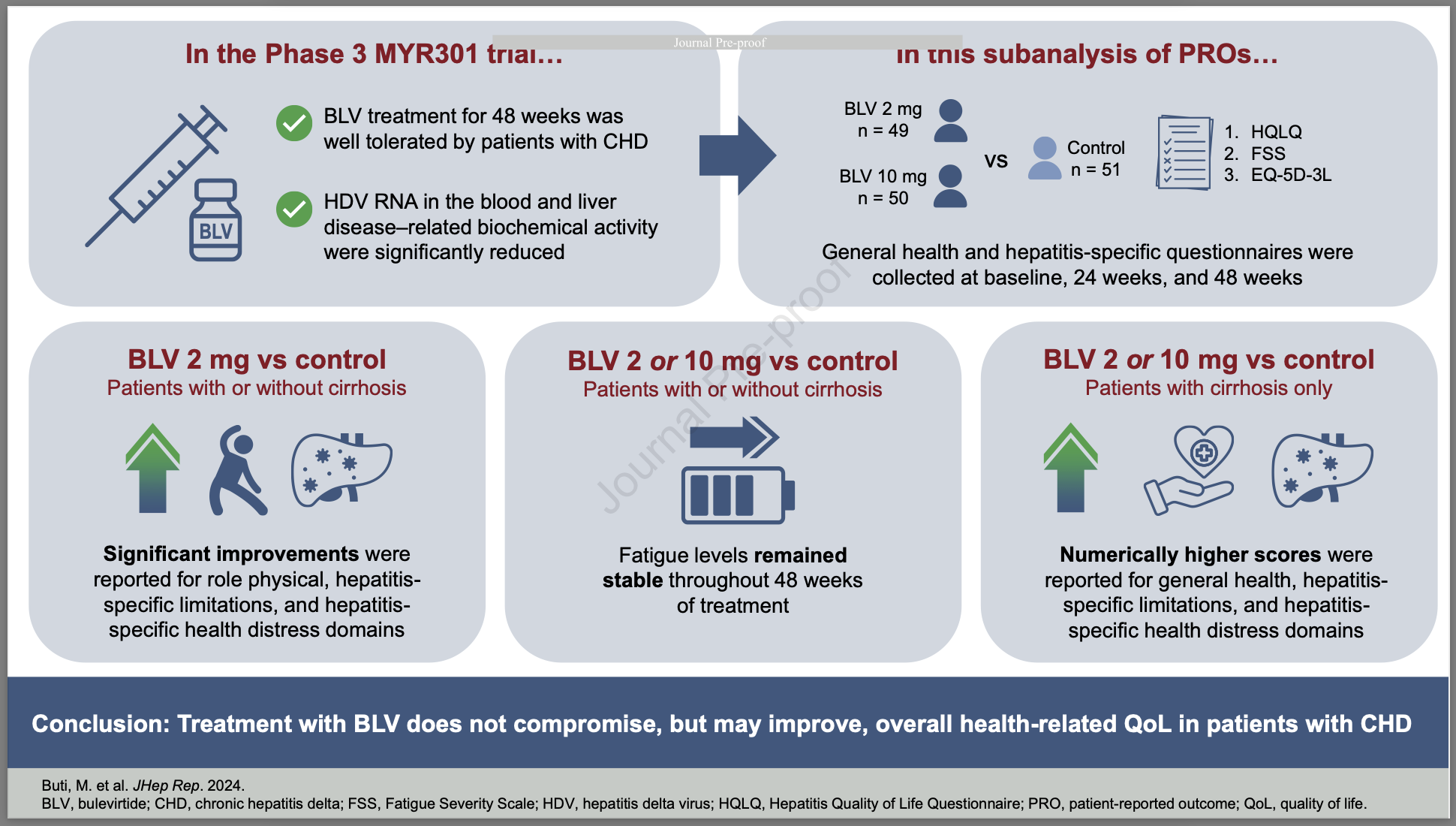
Highlights
- Bulevirtide (BLV) 2 or 10 mg is a new treatment for patients with chronic hepatitis delta (CHD)
- This study reports the first data on the health-related quality of life (HRQoL) of patients with CHD who received BLV
- Patients who received BLV vs no therapy for 48 weeks had improved physical and hepatitis-related QoL
- BLV 10 mg may improve physical and general HRQoL for patients with CHD who have advanced liver disease
- BLV was well tolerated, as supported by the maintenance and improvement of overall HRQoL
Abstract
Background & Aims
Once-daily treatment of chronic hepatitis delta (CHD) with bulevirtide is well tolerated and associated with significant reductions in HDV RNA in the blood and in biochemical liver disease activity. This study explored the effects of 48-week bulevirtide treatment on health-related quality of life (HRQoL) in patients with CHD.
Methods
In an open-label, randomised, Phase 3 trial, 150 patients with CHD and compensated liver disease were stratified by liver cirrhosis status and randomised 1:1:1 to no treatment (control), bulevirtide 2 mg/day, or bulevirtide 10 mg/day for 48 weeks. HRQoL was evaluated by the following patient-reported outcome (PRO) instruments at baseline, 24 weeks, and 48 weeks: EQ-5D-3L, Hepatitis Quality of Life Questionnaire (HQLQ), and Fatigue Severity Scale (FSS).
Results
Patient characteristics and HRQoL scores were balanced at baseline between the treatment (2 mg, n = 49; 10 mg, n = 50) and control (n = 51) groups. Patients receiving 2-mg bulevirtide reported significant improvements compared with controls on the HQLQ domains of role physical, hepatitis-specific limitations, and hepatitis-specific health distress. Numerically higher scores for general health, hepatitis-specific limitations, and hepatitis-specific health distress domains were reported by patients with cirrhosis who received bulevirtide vs control. FSS scores remained stable across treatment groups throughout. At week 48, patients in the 2-mg group showed greater mean improvement from baseline in health status compared with controls on the EQ-5D-3L visual analogue scale.
Conclusion
PROs indicate that 48-week treatment with bulevirtide monotherapy may improve aspects of HRQoL in patients with CHD.
Impact and implications
Bulevirtide 2 mg is the only approved treatment for patients with chronic hepatitis delta (CHD) in the EU. Patients with CHD have worse quality of life scores than those with chronic hepatitis B. Bulevirtide treatment for 48 weeks reduced HDV RNA and alanine aminotransferase levels and was well tolerated among patients with CHD. For the first time, this study shows that patients who received bulevirtide therapy for 48 weeks reported improvements in physical and hepatitis-related quality of life domains compared to those who did not receive therapy (control group).
