A call to reclassify the delta hepatitis virus as an orphan disease
A call to reclassify the delta hepatitis virus as an orphan disease
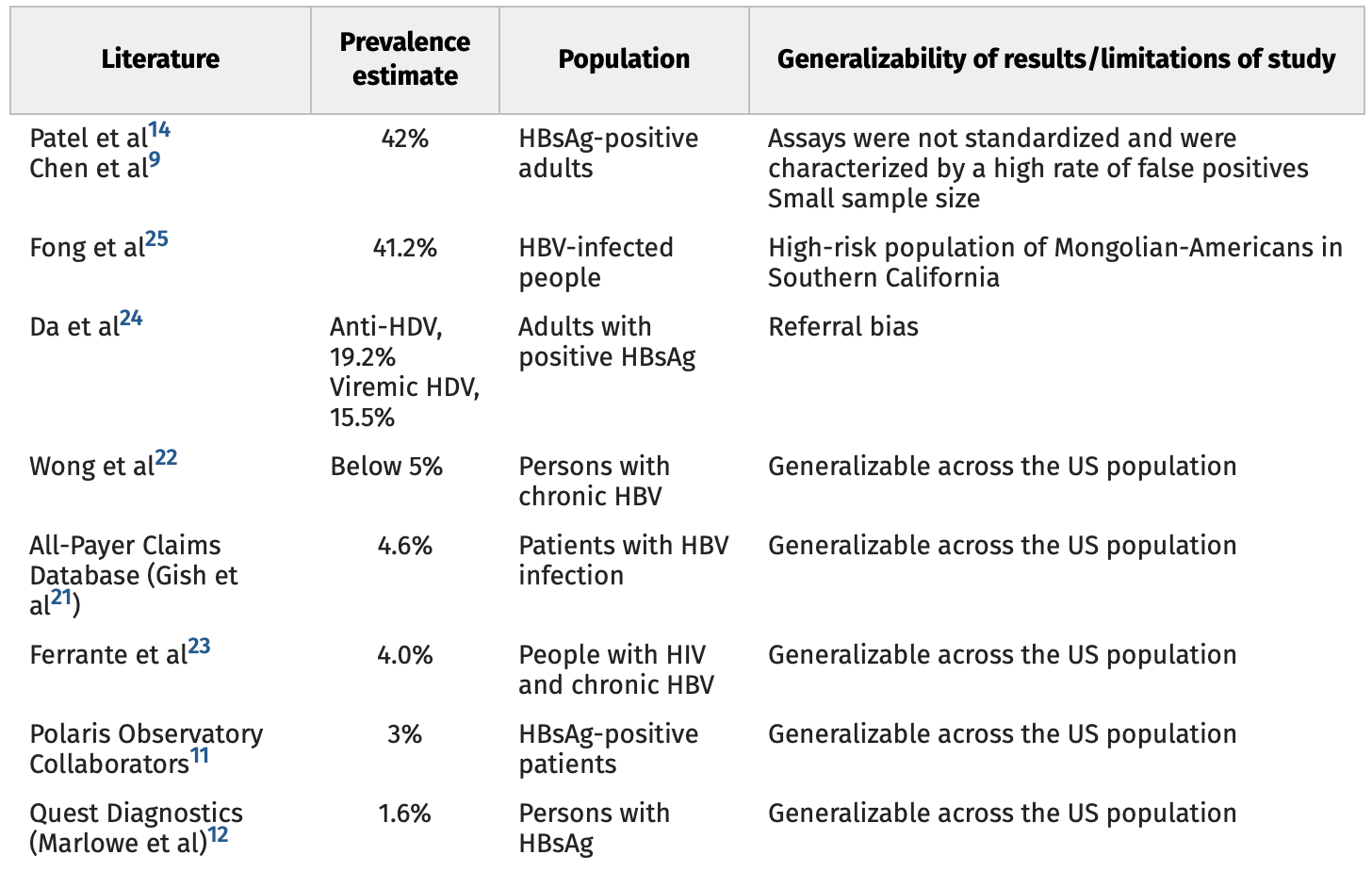
Chronic infection with HDV is considered the most aggressive and severe form of viral hepatitis, associated with an increased risk of cirrhosis and liver cancer. Due to high morbidity and mortality, there is a clear need for additional therapies for this disease. The Food and Drug Administration (FDA)’s Office of Orphan Products Development should continue to recognize chronic HDV as an orphan disease for current and future medication treatment applications. It is important to note that lonafarnib and myrcludex have previously received FDA orphan status designation. The Orphan Drug Act defines a rare disease or condition as one that affects <200,000 people in the United States. As outlined in this article, the great majority of the current and historical data support an orphan designation.
Despite causing severe liver disease, HDV remains largely neglected in research, testing, epidemiology, and public health policy settings. Because the Orphan Drug Act provides incentives to drug companies to research, develop, and distribute therapeutics for people with rare diseases, the designation of HDV as orphan status will open up the development of therapies for this virus and improve outcomes for patients with HDV.
