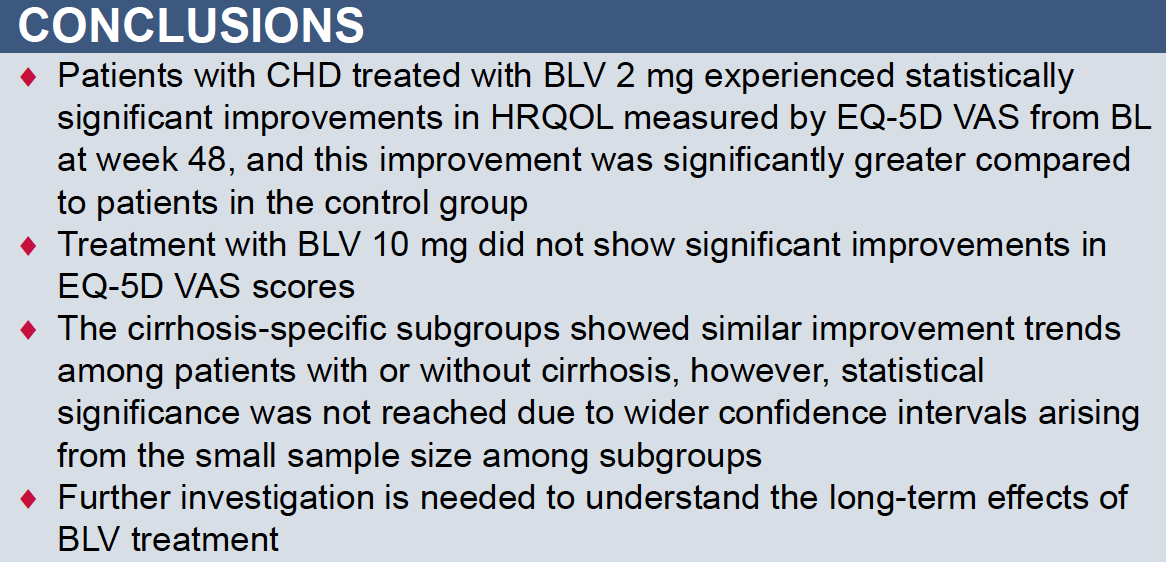
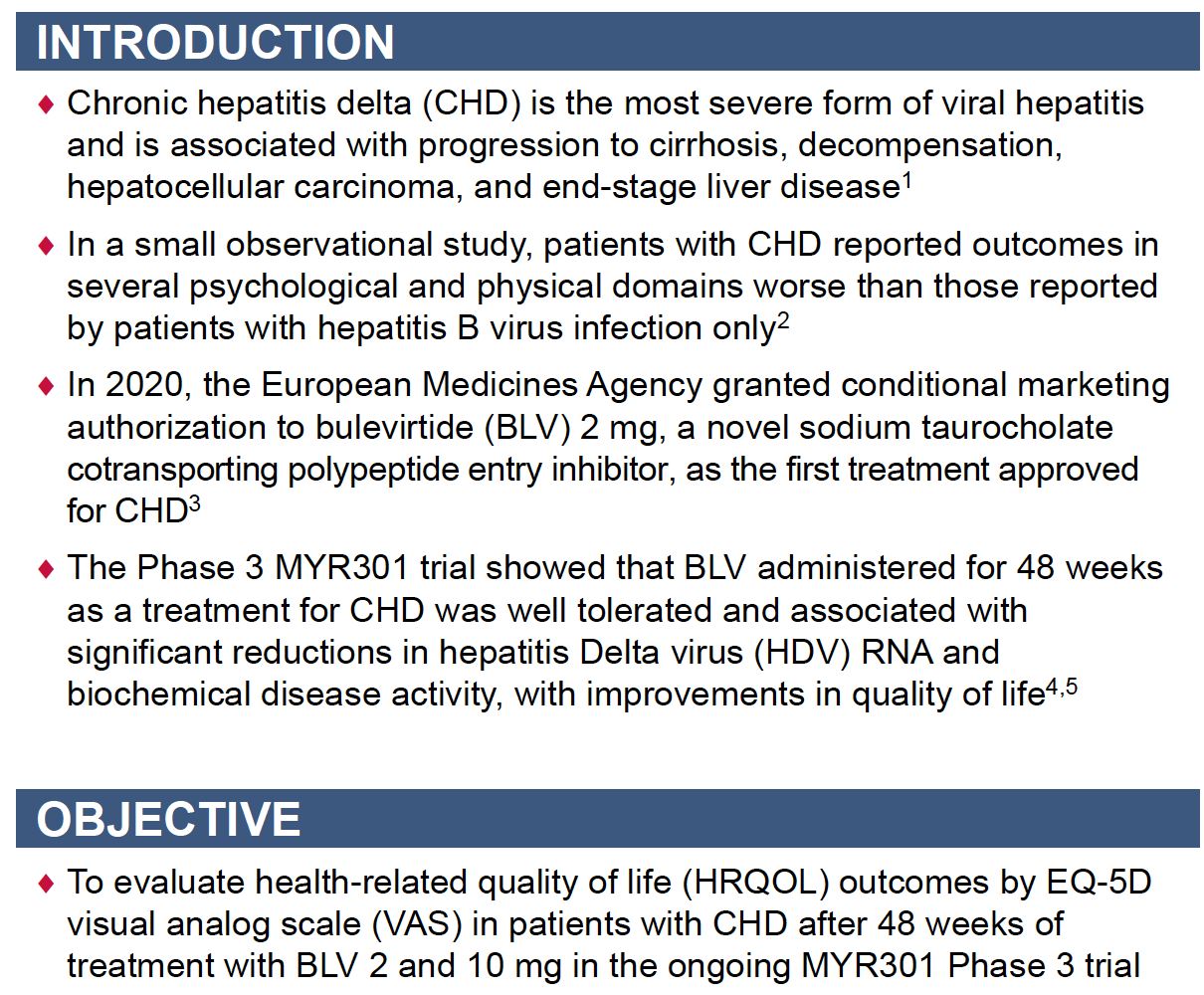
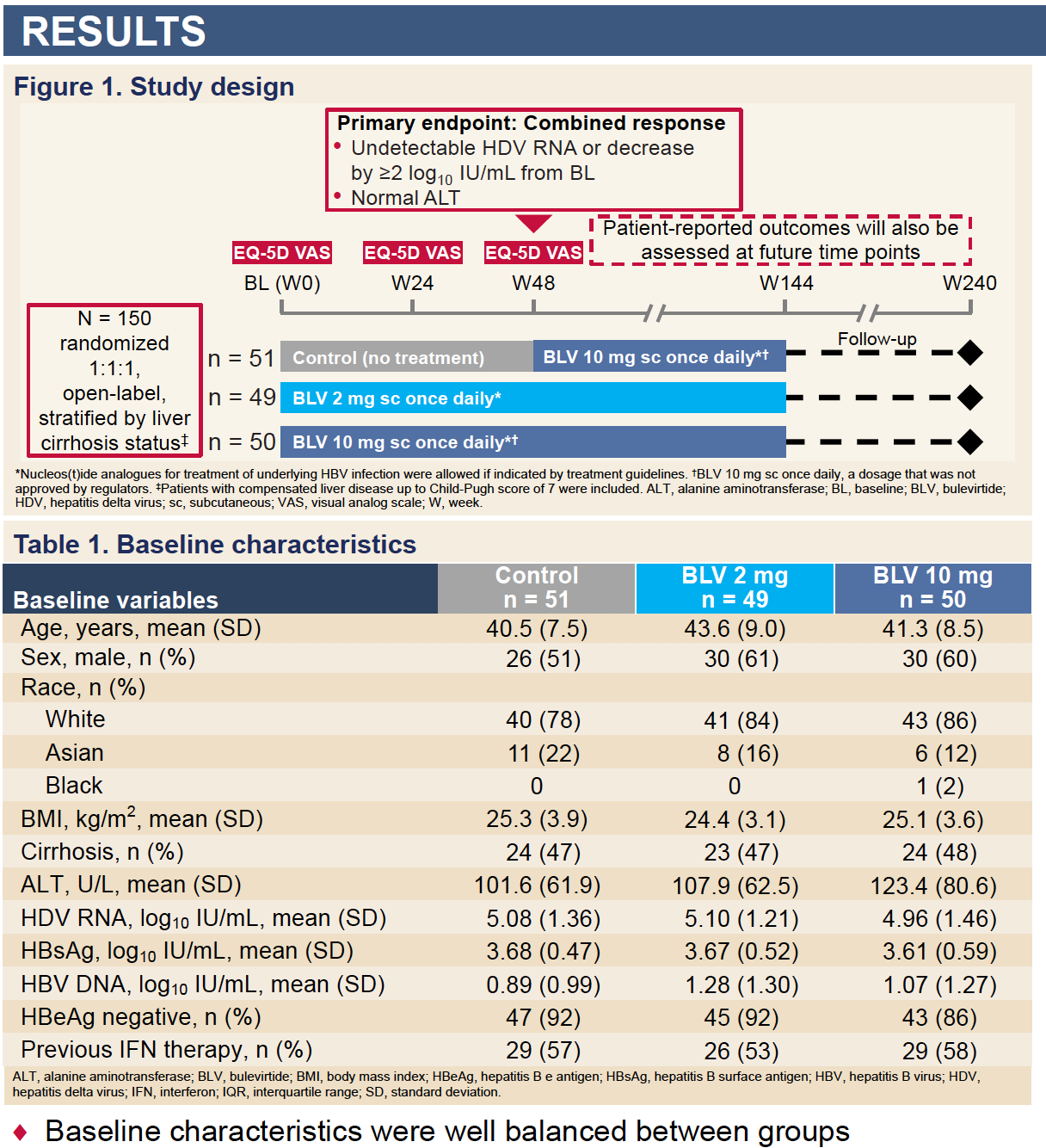
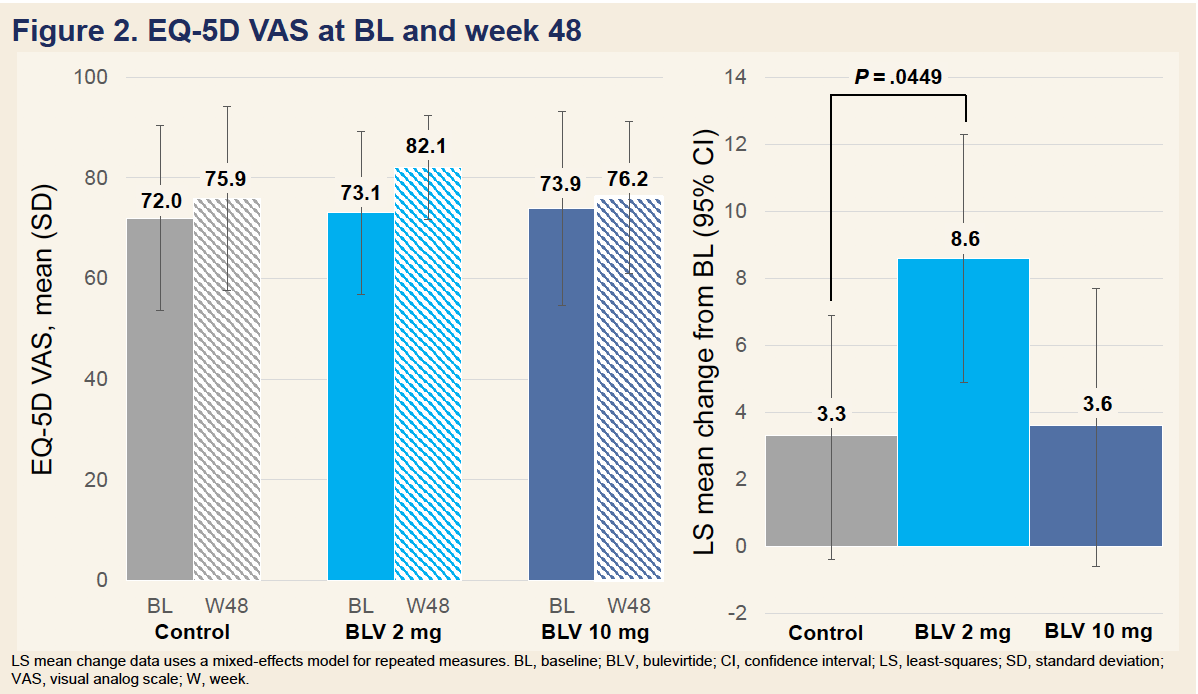
Bulevirtide Improves Health-Related Quality of Life
Bulevirtide Improves Health-Related Quality of Life Measured by EQ-5D VAS inPatients With Chronic Hepatitis Delta: An Exploratory Analysis of a Phase 3 Trial at 48 Weeks
AASLD nov 4-8 2022
Maria Buti1, Heiner Wedemeyer2, Soo Aleman3, Vladimir Chulanov4,5, Viacheslav Morozov6, Olga Sagalova7, Tatyana Stepanova8, Robert G Gish9, Andrew Lloyd10,
Ankita M Kaushik11, Vithika Suri11, Dmitry Manuilov11, Anu O Osinusi11, John Flaherty11, Shubhram Pandey12, Barinder Singh12, Pietro Lampertico13,14
1Hospital Universitario Valle Hebron, Barcelona, Spain; 2Medizinische Hochschule Hannover, Hannover, Germany; 3Karolinska Universitssjukhuset, Karolinska Institutet, Stockholm, Sweden; 4National Medical Research Center of Tuberculosis and Infectious Diseases, Ministry of Health, Moscow, Russian Federation; 5Sechenov University, Moscow, Russian Federation; 6Hepatolog, LLC, Samara,
Russian Federation; 7Southern Ural State Medical University, Chelyabinsk, Russian Federation; 8Clinic of Modern Medicine, Moscow, Russian Federation; 9Hepatitis B Foundation, Doylestown, PA, USA; 10Acaster Lloyd Consulting Ltd, London, UK; 11Gilead Sciences, Inc., Foster City, CA, USA; 12Pharmacoevidence Private Ltd, Punjab, India;
13Foundation Irccs Ca’ Granda Ospedale Maggiore Policlinico, Division of Gastroenterology and Hepatology, Milan, Italy; 14CRC “A. M. and A. Migliavacca” Center for Liver Disease, Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy

References: 1. Lempp FA, et al. Nat Rev Gastroenterol Hepatol. 2016;13:580–89. 2. Buti M, et al. JHEP Rep. 2021;3:100280. 3. Hepcludex Assessment Report. European Medicines Agency; 2020. 4. Wedemeyer H, et al. J Hepatol. 2022;77(S1):S4. Abstract GS006. 5. Buti M, et al. J Hepatol. 2022;77(S1):S4. Abstract OS149. Acknowledgments: This study was funded by Gilead Sciences, Inc., Foster City, CA, USA. Medical writing and editorial support were provided by Danielle Shepherd, PhD, AlphaScientia, LLC, San Francisco, CA, USA, and funded by Gilead Sciences, Inc. Disclosures: MB declares financial relationships with Gilead Sciences, Inc., and AbbVie for speaking and teaching. HW reports Shubhra honoraria for speaking or consulting from Abbott; AbbVie; BMS; Boehringer Ingelheim; Eiger; Gilead Sciences, Inc.; Janssen; Merck Sharp & Dohme; MYR GmbH; Novartis; Novira; Roche; Roche Diagnostics; Siemens; and Transgene; and research support from Abbott; BMS; Gilead Sciences, Inc.; Novartis; Roche Diagnostics; and Roche. SA reports speaking honoraria from Gilead Sciences, Inc; AbbVie; Merck Sharp & Dohme; and Biogen; and research grants from Gilead Sciences, Inc. and AbbVie. VC reports consultancy and/or speaker’s bureau from Abbott; AbbVie; AstraZeneca; BMS; Gilead Sciences, Inc.; GSK; Hepatera; MSD/Merck; Pharmstandard; Roche; and R-Pharm. VM reports no conflicts of interest. OS reports honoraria for speaking or consulting from: AbbVie; Gilead Sciences, Inc.; Merck/Schering-Plough; Abbott; Pharmstandart; and Hepatera. TS reports no conflicts of interest. RG reports grants from Gilead Sciences, Inc.; consultancy and advisory for Abbott; AbbVie; Altimmune; Antios; Arrowhead; Dynavax; Eiger; Eisai; Enyo; Fibronostics, Inc.; Fujifilm Wako Diagnostics; Genentech; Genlantis; Gerson Lehrman Group; Gilead Sciences, Inc.; Helios; HepaTX; HepQuant; Intercept; Janssen; Merck; Perspectum; Pfizer; Quest; Sonic Incytes; Topography Health; and Venatorx; scientific or clinical advisory board for AbbVie; Antios; Dynavax; Enyo; Genentech; Genlantis; Gilead Sciences, Inc.; Helios; HepaTX; HepQuant; Intercept; Janssen; Merck; Pfizer; and Prodigy; chair clinical advisory board for Prodigy; clinical trials alliance with Topography Health; data safety monitoring board for Altimmune; Arrowhead; CymaBay Therapeutics, Inc.; and Durect; speakers bureau for AbbVie; Bristol Myers Squibb; Eisai; Genentech; Gilead Sciences, Inc.; and Intercept; minor stock shareholder in RiboSciences and CoCrystal; and stock options for AngioCrine; Eiger; Genlantis; HepaTX; and HepQuant; AL is an employee of Acaster Lloyd Consulting Ltd, London, UK, which was contracted to support this research. AK, VS, DM, AO, and JF are employees of Gilead Sciences, Inc., and may own stock or stock options. SP and BS are employees of Pharmacoevidence and report no other conflicts of interest. PL reports speaking and teaching fees from and participation in advisory committees or review panels for AbbVie; Aligos; Alnylam; Antios; Arrowhead; BMS; Eiger; Gilead Sciences, Inc.; GSK; Janssen; MSD; MYR GmbH; Roche; and Spring Bank Pharmaceuticals.
