Duration of Nucleos(t)ide Analogue Treatments
Duration of Nucleos(t)ide Analogue Treatments in Patients With Chronic Hepatitis B Virus Infection in the United States
ABSTRACT
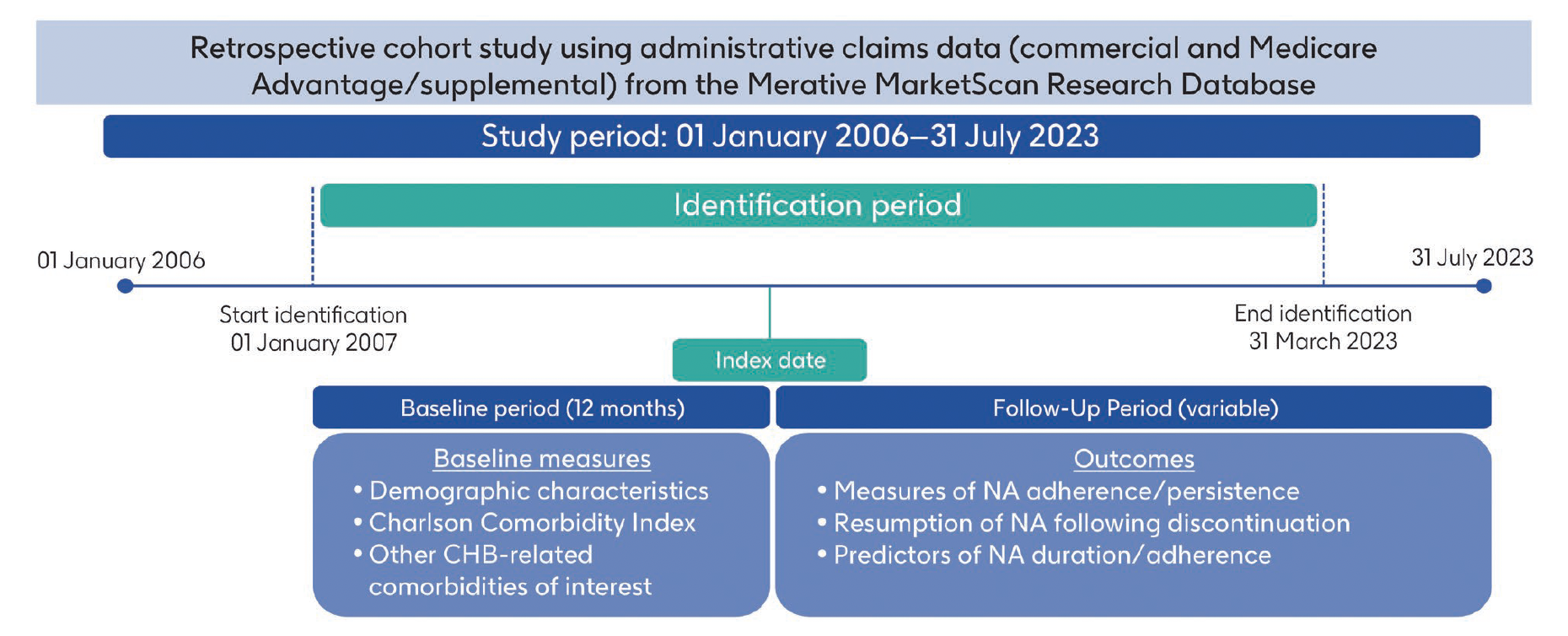
Viral hepatitis caused by hepatitis B virus accounts for a significant disease burden. Nucleos(t)ide analogues (NAs) are the standard of care for chronic hepatitis B (CHB) infection; however, treatment is long-term, and viral eradication resulting in cure is rare. Adherence to NAs is vital for disease control. Here, we describe real-world treatment patterns among adult patients with CHB infection initiating second-generation NAs in the United States. This retrospective cohort study used United States administrative claims data. From the January 1, 2006 to July 31, 2023 period, we identified patients aged ≥ 18 years diagnosed with CHB infection who initiated second-generation NAs. Patient characteristics and real-world NA utilisation measures were reported, including time to discontinuation, resumption of NA treatment, adherence and predictors of adherence. In total, 6696 patients met the study eligibility criteria. Mean age was 47.2 (standard deviation: 11.5) years, and 41.6% of patients were female. The most common index NA treatments were tenofovir alafenamide (48.5%) and entecavir (41.7%). Median follow-up duration was 24.4 months. Overall, 40.6% of patients discontinued treatment; discontinuation probability was 29.4% at 12 months and 55.6% at 5 years. Of those who discontinued, 45.7% restarted during the study period. Mean adherence (proportion of days covered [PDC]) was 0.91, and 86.5% of participants had a PDC ≥ 80%. This study highlights the challenge of long-term persistence with NA treatment. An unmet need in CHB infection management is novel treatments with finite durations that offer an opportunity to achieve cure and mitigate disease progression.
